Accreditations
Dr J. C. Stoltz
INTRODUCTION
Quality management is a challenging topic with different views on what to do and how to do it (Knowles, 2011), and in order for companies to make sense of it all, impartial guidance is required, since Knowles (2011) warns that quality standards are for guidance purposes and not a manual on how to do things.
More than 25 years of research have been conducted on the various ISO (International Organization for Standardization) standards and their impact on organisations. One of the most popular standards is the ISO 9001 Quality Management Standard, which is the focus of this research since ISO 9001 is used in over 175 countries and the number of certifications is increasing (Hallberg et al., 2018).
Quality is an important subject in the modern business world (Dahlgaard, et al., 2007). Total Quality Management (TQM) and the ISO-based Quality Management System (QMS) have been two of the most common approaches utilised by companies since
the 1980s (Chen et al., 2016). According to Chen et al. (2016) empirical evidence indicates that both TQM and QMS are difficult to implement and sometimes they conflict with each other.
Irrespective of the specific theme or topic being researched regarding ISO 9001 certification, the findings are generally conflicting, unclear and usually subjective. The main themes in academic articles associated with ISO 9001 certification are the motivation for ISO implementation, management involvement, customer satisfaction, financial impact and operational performance.
On 9 December 2016, regulations relating to Medical Devices (MD) and In Vitro Diagnostic (IVD) Medical Devices in particular, were published by the South African Minister of Health in Government Gazette 40480, Government Notice 1515 (Government Gazette, 2016). In terms of Regulations 5 and 8, for purpose of both licensing and registration, companies are required to submit proof of certification to a Quality Management
System “as determined by the South African Medicines Control Council (MCC). This publication, therefore, made it mandatory for all Medical Device companies including In Vitro Diagnostic companies in South Africa (SA) to be ISO certified.
With the introduction of mandatory ISO certification within the IVD industry in SA, and added to this a specific Quality Management System for Medical Devices (ISO 13485, 2016), the value, benefits and sustainability of the change in the quality criteria with regard to ISO certification in the IVD industry in SA needed to be assessed. A decision was therefore taken in 2014 to expand the role of the MCC in SA to include regulation of IVDs in preparation for the creation of the South African Health Products Regulatory Authority (SAPHRA). These new stringent policies have caused a decrease in investments in research and development of new products by medical device manufacturers (Mordor Intelligence, 2019) and dampening innovation and hindering growth in the IVD market.
The South African Health Products Regulatory Authority (SAHPRA) describes in vitro diagnostics (IVD) as “a medical device, used alone or in combination, intended by the manufacturer for the in vitro examination of specimens derived from the human body solely or principally to provide information for diagnostic, monitoring or compatibility purposes” (SAHPRA, 2019). The Compound Annual Growth Rate (CAGR) for the in vitro diagnostic market in South Africa is forecasted at 7.21% by 2024 (Mordor Intelligence, 2019). This predicted growth can be attributed to high-burden chronic diseases like cancer, infectious diseases and diabetes. The Southern African Laboratory Diagnostic Association (SALDA) is a collaboration of multinational and local companies that distribute IVD tests. A total of 47 local and multinational IVD companies are members of SALDA, and are responsible for manufacturing, importing, selling, servicing, marketing and distributing IVD products in South Africa.
The International Organisation for Standardization (ISO) is a worldwide federation of national standards bodies that was developed in 1987. The ISO standard provides the fundamental concepts and principles that are to be utilised when creating a quality management system according to the requirements in the ISO standard (Abuhav, 2017). The ISO documents provide guidelines, requirements and specifications to be used consistently to ensure that processes, products and services are appropriate for their purposes. ISO was founded in 1947 and is the major developer of voluntary International Standards and since its inception, it (ISO) has published 22,955 International Standards covering most aspects of business and technology.
A standard is a set of guidelines or specifications to ensure that a product or service does what it is supposed to do (Kenya National Secretariat, 2016). A Quality Standard can be defined as a document that provides requirements, specifications or guidelines that can consistently be used to ensure products, services and processes are fit for their intended use. According to the American Society for Quality (2021), successful companies recognize quality standards as business tools to be managed
alongside other policies. According to Merih (2016), quality is one of the most important facets in a business and it (quality) has different meanings for different people, as it can echo several different realities based on the terms used. Merih (2016) further states that quality is merely meeting the requirements of the customer or adhering to the minimum specification of a product or service delivered.
One of the fundamental flaws with regard to quality in South Africa is registering with an ISO quality system merely to meet customer demands (Ramdass and Nemavhola, 2018). Obtaining certification may provide some benefits, like greater market share, but sadly other important principles of the quality system like process approach and evidence-based decision making are ignored. One common thread in the progression of quality management is that attention to quality has moved progressively further up in the organisation hierarchy. Originally, quality was the responsibility of the line worker, later on the inspector, then the supervisor, then middle management and of late, upper management. In recent years this has changed to include customer orientation as this is the initial point on the activities chain that quality depends on (Popescu, et al., 2017).
Given today’s competitive business environment, companies have been compelled to pursue quality and quality management systems to enhance their competitive advantage. This is no different in the medical field since according to Alzoubi et al. (2019), the healthcare sector has been concerned about quality for more than a decade. Aggarwal et al. (2019) state that Quality management in healthcare is a critical requirement and urges competent authorities to enforce the responsibility for ensuring high-quality standards and quality in the healthcare arena.
In light of the above, the aim of the research is to develop a conceptual model to depict the relationship between ISO certification as a quality management strategy in the IVD Industry in SA and various possible benefits as espoused in the literature.
LITERATURE REVIEW
In-Vitro Diagnostics
The food and drug administration (FDA) defines in vitro diagnostic (IVD) products as reagents, instruments and systems intended for use in the diagnosis of diseases or other conditions in order to cure, mitigate, treat or prevent a disease or its sequelae. Specimens taken from the human body are tested on IVD products. The specimen derived from the human body is solely to provide information for diagnostic monitoring or compatibility purposes (World Health Organization, 2017).
The World Health Organisation (WHO) defines a medical device as any instrument, appliance, implant, apparatus, reagent or material used alone or in combination with human beings for a specific medical purpose. IVDs differ from medical devices in that they do not come into direct contact with the patient. IVDs accomplish their role based on evidence that they provide, compared to medical devices that are in contact with or implanted into the body. IVDs allow for easier and faster clinical diagnosis that is less painful to patients.
Figure 1: South Africa in vitro diagnostic market
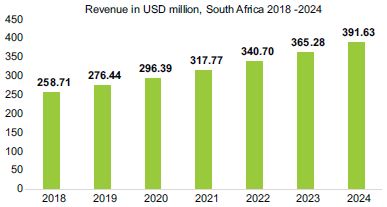
Source adapted from: Mordor Intelligence (2019)
. The drive for IVD development is the prevention and early intervention of diseases (Zhou et al., 2015). IVDs are also used to assess the likelihood of developing a disease and to guide patient management (Rohr, et al., 2016). Figure 1 reflects that the South African IVD market revenue in US dollars (USD) from 2018 to 2024 projects a compounded annual growth rate (CAGR) of 7.21% is expected over the forecast period.
The major influences that can be linked to the growth in the IVD industry in South Africa are the high burden of chronic diseases which creates a high demand for diagnostics. South Africa is seeing an increase in non-communicable diseases (NDCs) which include heart diseases, diabetes, chronic lung disease, stroke and cancer. The favourable growth forecast for the IVD market is based on the advancement of IVDs that provide new tools to support the increase in disease diagnosis and treatment.
In the absence of a regulatory authority in South Africa, the industry relied on the National Health Laboratory Service (NHLS) to provide advice and guidance. This however changed in 2014 when the Medicines Control Council (MCC) included IVD’s in preparation for the creation of the South African Health Products Regulatory Authority (SAHPRA) which was established in February 2018. SAHPRA ensures that medicines, medical devices and IVDs meet the mandatory standards to safeguard the health and well-being of South Africans.
SAHPRA was created by the South African Government as an entity of the National Department of Health and assumed the responsibility of both the MCC and the Directorate of Radiation Control (DRC). Recently SAHPRA was constituted as an independent entity that reports to the National Minister of Health through its Board. According to Mordor Intelligence (2019), the strict policies will result in a reduction of investments by manufactures in research and development and could hamper innovation.
The Southern African Laboratory Diagnostic Association (SALDA) is a collaboration of multinational and local companies that distribute IVD tests. A total of 47 local and multinational IVD companies are members of SALDA, and are responsible for manufacturing, importing, selling, servicing, marketing and distributing IVD products in South Africa.
Quality Management in Healthcare
Due to the complexity and uniqueness of health services, they have taken longer than other industries to adopt and accept quality management systems. ISO 9001 certifications are becoming more popular in the healthcare industry and the interest accelerated since 2008. The reason behind this is that ISO 9001 certification and hospital accreditation are essential instruments to improve the overall quality of the health service.
During 1946 in London, 25 countries met to discuss the future in International Standardisation that led to ISO officially being established during 1947. It started with 67 technical committees that expanded over the years to be 781 technical committees from 164 countries. The first ISO 9001 standard was officially introduced in 1987 and since then several updates have been done to the standard (Ramphal, 2015), the first update transpired in 1987. The standard ISO 9001:1987 had a military tone of conformity and the procedures were most suitable for the manufacturing industry. Two further ISO 9000 standards were updated, ISO 9002 for companies with manufacturing capabilities and ISO 9003 for distributors. According to Reid (2015), the scope of the quality management system was designed to include the entire organisation and not just essential functions or departments.
The second ISO 9001 update occurred in 1994 and ISO 9001:1994 was created. The main drive of this revision was to move quality management from a remedy perspective to a more preventative perspective. The product was checked and monitored during every stage of manufacturing to delivery rather than only evaluating the finished product. The ISO 9001:2000 version announced the concept of process management as the main driver of the standard and required a documented system compared to a system of documents. The aim was to create an integrated goal within the organisation and this was achieved by a set of eight core quality management principles, namely improved consistency with traceability; enhance customer focus; focus leadership; the involvement of people; the system approach to management; continual improvement; a factual approach to decision making and mutually beneficial supplier relationships.
ISO 9001 (2008) had negligible changes with an attempt to provide a better understanding on the quality requirements and to maintain uniformity and encourage integration with other standards such as ISO 14001:2004 which is the standard for environmental management.
The changes in the last ISO 9001 update labelled ISO 9001:2015, introduced risk-based thinking with fewer prescribed requirements. There was increased emphasis on organisational context and leadership requirements were also increased. The 2015 update emphasised desired outcomes in order to improve customer satisfaction.
ISO 13485 is an industry specific interpretation of ISO 9001 with emphasis on the medical device and in vitro diagnostic industry. ISO 9001 and ISO 13485 are meant to be generic and can be applied to all kinds of companies regardless of the type or size. Even though ISO 13485 specifies a sector specific management system, many of the ISO 13485 requirements are applicable to ISO 9001.
ISO 13485 relates to companies manufacturing medical devices and providing related services. ISO 13485 must satisfy specific requirements and can be used as a basis for the development, production and installation of medical devices. The aim is for a company to demonstrate the ability to offer medical devices and related services that constantly meets the requirements of the customer and more importantly, meet the applicable regulatory requirements for medical devices (Green, 2005). The standard reflects the current regulations in an aim to harmonise worldwide medical device regulations. According to the ISO Survey, the number of ISO 9001 and ISO 13485 certifications in South Africa for all industries are indicated in Table 1.
Figure 2 reflects ISO 9001 certifications worldwide up to 2017, as well as each continent’s contribution in percentage terms to certifications worldwide. South Africa represented 37.9% of the Africa continent certifications. Europe and East Asia/Pacific, combined, have 85.3% of the world’s ISO 9001 certifications.
With the introduction of forced ISO certification within the IVD industry in South Africa, and added to this a specific Quality Management System for Medical devices (ISO 13485:2016), the value, benefits and sustainability of the change in the quality criteria with regards to ISO certification in the IVD industry in SA is a concern. The purpose of ISO is strategic in nature and this is confirmed in clause 4.1 of the ISO 9001:2015 quality management system requirements, which states that the organisation shall determine external and internal matters that are relevant to its purpose and its strategic direction, and that affect its ability to achieve the intended results of its quality management system.
Table 1: Number of ISO certifications in South Africa

Source adapted from: ISO Survey (2018)
The South African Department of Health stated that companies must provide the SAHPRA with evidence of their Quality Management System certification. This means that all IVD companies must have ISO certification.
Figure 2: ISO 9001 certifications worldwide
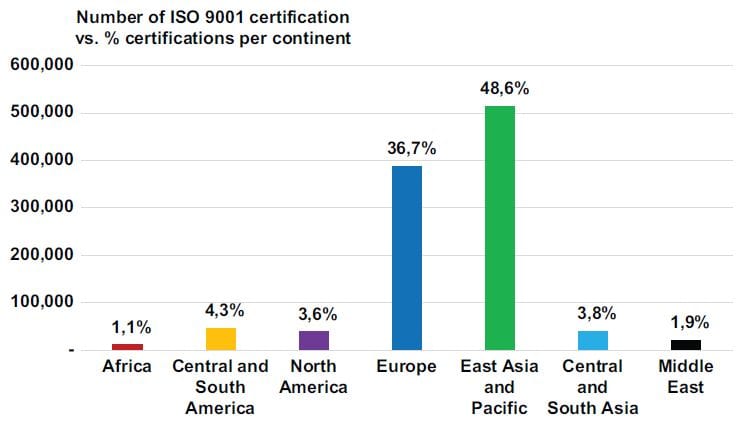
Source adapted from: ISO Survey, (2018)
In 2019, only 45% of IVD companies operated with ISO certification (Maseko, 2019). To implement ISO and obtain certification without any prior experience is not easy and it is very costly. Many resources are required and the timeline for implementation is very short. Many organisations decide not to implement ISO certification due to various reasons. Figure 3 reflects the reasons why companies do not get certified.
Quality and the IVD Industry
More than 25 years of research has been conducted on quality management with the main themes identified as implementation, performance, cost, motivation and customers service. To date no research has been performed on the IVD industry and quality and/ or certification.
Little information is currently available on why some South African IVD companies are ISO certified and some are not. It is also unclear what contribution ISO certification makes to the South Africa IVD medical device industry and customers’ perceptions regarding ISO-certified and non-certified companies. Moreover, the impact of compulsory ISO certification on the IVD industry in South Africa is also not documented.
With the mandatory implementation of a Quality Management System within each IVD company, the research will critically evaluate ISO certification in the IVD Industry in South Africa and propose a conceptual model to manage it. One of the most popular standards is the ISO 9001 Quality Management Standard, since it is used in over 175 countries and the number of certifications is increasing (Hallberg et al., 2018). The motivation by organizations to implement ISO may be internal and/or external. Internal motivations include improving efficiencies, communication and delivery times, motivating personnel and gaining a competitive advantage. External motivations include gaining access to new markets and tender business, improving corporate image and customer satisfaction, and increasing market share.
Figure 3: Reasons for non-certification
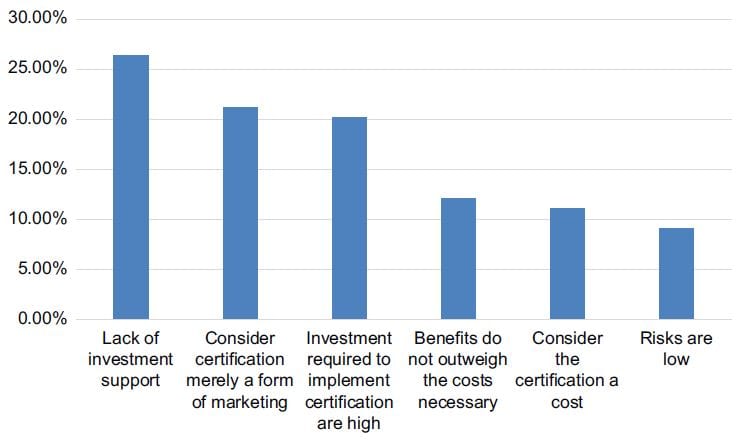
Source: Adapted from Santos et al., (2016)
Valmohammadi and Kalantari argued that internal motivations such as cost reduction, quality improvement and employee knowledge significantly affected the performance of manufacturing companies. Djofack and Camacho (2017) found that internal motivation such as improved procedures were the main reason for ISO certification. Internal motivation for ISO 9001 implementation also positively influence continuous improvement within companies. The higher the level of motivation for implementation, the greater the adherence of the standard, which will yield better results.
Allur and Hera-Saizarbitoria (2014) stated that internal motivations for ISO implementation had no effect on internal procedures and argued that benefits such as productivity and improved services were limited in the long run. Furthermore, external motivation for implementation drives companies to focus on enhancing their quality reputation and reacting to external pressures. Motivation for ISO 9001 implementation, whether internal or external, determines the success and benefits obtained from ISO 9001 certification.
Quality Management
Managers are responsible for ensuring awareness and understanding of the ISO 9001 system throughout the company and for supporting the implementation process. Management commitment throughout the implementation process is imperative. Managers should work closely with employees by showing their commitment, and realistic goals should be set concerning implementation timelines, costs and results.
Managers’ understanding of the ISO 9001 quality system, customer demands and the company’s strategic direction for servicing the customer would significantly enhance the effectiveness of the ISO 9001 system and create benefits, e.g. improved customer service. Cho et al. (2017) argued that although ISO 9001 contributed to improving the general operating performance of a company, the greatest improvements came from top management’s involvement with ISO 9001 implementation and from maintaining the quality system.
Management goals and objectives that integrate the quality system and business goals enable the implementation and successful maintenance of the system thereafter. Top management support also significantly aids the continuous improvement of the ISO 9001 quality system.
One of the main principles of ISO 9001 is the focus on customer service. According to Valmohammadi and Kalantari (2015), ISO 9001 certified companies showed better levels of performance than non-certified companies as ISO 9001’s quality management system requirements helped to meet customers’ requirements and demands.
ISO 9001 helped companies better understand and manage business relationships that would increase customer service (Hallberg et al., 2018). This is achieved with tools like a SWOT analysis (strengths, weakness, opportunities and threats) and risk-based thinking (ISO, 2015). Nabavi et al. (2014) agreed with these findings and stated that ISO 9001 certification significantly boosted customer
satisfaction and increased competitive advantage, notwithstanding the increase in the costs associated with achieving this.
Martin (2017) contended that customer satisfaction in any business industry was positively affected by ISO 9001 certification by reason that operational performance would increase if resources were managed properly. Shaharudin et al. (2018) disagreed, arguing that only employee attributes drove customer satisfaction as companies were more concerned about internal processes than external perspectives received by customers. With reference to research within the healthcare service sector, findings regarding the effectiveness of ISO 9001 certification and customer satisfaction are inconclusive (Rakhmawati, et al., 2014).
The literature reviewed indicates contrasting views on how ISO 9001 certification affected companies’ financial performance. Some studies have indicated that ISO 9001 certification positively influenced a company’s financial performance (Ochieng et al., 2015), and the increase in financial performance due to ISO 9001 has been strongly validated in other studies (Psomas and Antony, 2015). However, many companies that lost their ISO certification saw no change in their financial performance based on the return on assets (ROA) and return on sales (ROS) that were used as the statistical measures at the time.
Studies conducted in Greece maintained that ISO 9001 was closely associated with a company’s financial performance (Chatzoglou et al., 2015) and research in Romania found that ISO 9001 increased overall financial performance (Ionașcu, et al., 2017).
In contrast to the above findings, Kafel and Simon (2017) found no correlation between ISO certification and financial performance. There is no clear evidence that ISO alone affects the financial performance of a company; nor do profits increase after ISO 9000 certification (Zondo, 2018). Several studies concluded that claims regarding improved financial performance owing to ISO 9001 certification were inconclusive and unclear.
Considering the nature of ISO 9001, the processes implemented should increase business and operational performance if applied correctly. If all processes were streamlined, ISO should have a positive effect on a company’s overall performance (Galetto et al., 2017). ISO 9001 improved quality management practices in companies which would directly and significantly affect business performance (Patyal and Koilakuntla, 2017).
Some researchers are of the view that a major benefit of ISO certification was operational improvement (Kakouris and Sfakianaki, 2019). However, not all researchers agree with this, since according to Neyestani and Juanzon, an improvement in organisational performance after ISO 9001 implementation was inconsistent and unclear. Allur and Heras-Saizarbitoria (2014) stated that benefits derived after ISO 9001 certification were limited, and Aba et al. (2016) found that performance improvements due to ISO 9001 certification were limited. One explanation for these limitations was that companies did not take advantage of the strategic benefits after ISO 9001 certification.
The benefits of ISO 9001 certification include streamlining internal operations, entering new markets and scaling up operations. W (ISO, 2014) ilcock and Boys supported the finding that ISO 9001 certification offered a promising avenue to foster business development and business sustainability. Other benefits obtained from ISO 9001 certification were improvement of company image, access to tenders, access to new markets, compliance with legislation and an organised work environment. The main difficulties with ISO 9001 implementation were high certification costs, staff motivation and a change in company culture. Benefits would not be derived without the necessary resources, e.g. internal auditors and staff who have received training in ISO 9001 principle.
Customer Service
One of the main principles of ISO 9001 is the focus on customer service. The primary focus of quality management is to meet customer requirements and to strive to exceed customer expectations. Sustained success is achieved when a company attracts and retains the confidence of customers. It is important to understand the current and future needs of customers as every interaction with customers provides an opportunity to increase value for that customer.
Martin (2017) contended that customer satisfaction in any business industry is positively affected by ISO 9001 certification by reason that operational performance would increase if resources were managed properly. Shaharudin et al. (2018) disagreed, arguing that only employee attributes drove customer satisfaction as companies were more concerned about internal processes than external perspectives received by customers. With reference to research within the healthcare service sector, findings regarding the effectiveness of ISO 9001 certification and customer satisfaction are inconclusive. The difference in service delivery and customer satisfaction between ISO certified IVD companies and non-ISO certified IVD companies must be determined, if indeed any difference exists.
Against the above discussion, it may be postulated that there is a positive relationship between ISO certification of IVD companies in SA and customer service offered by these companies.
Organisational Performance
An important part of ISO certification is to establish a performance monitoring, measurement and analysis framework to determine the achieved performance levels and to determine whether those achieved levels are satisfactory to the company or not (Gokpinar, et al., 2019). Organisational performance measurement is critical for organisational effectiveness (Al- Damen, 2017). Some researchers are of the view that a major benefit of ISO certification is operational improvement. However, not all researchers agree, since according to Neyestani and Juanzon, an improvement in organisational performance after ISO 9001 implementation was inconsistent and unclear. Allur and Heras-Saizarbitoria (2014) stated that benefits derived after ISO 9001 certification were limited, and Aba et al.(2016) found that performance improvements due to ISO 9001 certification were limited. One explanation for these limitations is that companies
do not take advantage of the strategic benefits after ISO 9001 certification.
One can argue that ISO certification forces Quality Management practices which in turn influences quality performance, and quality performance has a direct and significant impact on business performance (Patyal and Koilakuntla, 2017). The level of success of ISO certification that leads to an increase in operational and business performance depends on the link between quality aspects and commitment of staff at all levels within the company (Ahmed, 2017).
What needs to be determined is whether ISO certification automatically guarantee an improvement in organisational performance or are there any other factors that contribute to a change in organisation performance, if any? Thus, it may be postulated that there is a positive relationship between ISO certification of IVD companies in South Africa and the organizational performance of these companies.
Product Quality
ISO 13485 is an industry specific interpretation of ISO 9001 with emphasis on the medical device and in vitro diagnostic industry. Even though ISO 13485 specifies a sector specific management system, many of the ISO 13485 requirements are applicable to ISO 9001. ISO 13485 relates to companies manufacturing medical devices and providing related services. ISO 13485 must satisfy specific requirements and can be used as a basis for the development, production and installation of medical devices. The aim is for a company to demonstrate the ability to offer medical devices and related services that constantly meets the requirements of the customer and more importantly, meet the applicable regulatory requirements for medical devices. The standard reflects the current regulations in an aim to harmonise worldwide medical device regulations.
IVD products have been supplied to the South African market for decades, either directly imported from other countries or distributed by local South Africa companies. Many products that have been manufactured and supplied will not change at all except for a label requirement here and there. How will ISO 13485:2016 certification change or guarantee the quality of products supplied to the South African market is still largely unexplored. Given the above, it may be postulated that there is a positive relationship between ISO certification and the quality of IVD products in South Africa.
Management Commitment
Leaders are responsible for quality management system effectiveness, but a perpetual complaint of quality professionals has been that they experience a challenge in communication with top management. One of the main barriers to implement a quality management system is the lack of support from leadership (Abd- Elwahed and El-Baz, 2018). According to Mosadeghrad (2015), there is a strong relationship between leadership and the success of total quality management. The success of the management system rests largely on the managers’ ability to create a vison and plan to drive organisational change to obtain quality management success.
Figure 4: Conceptual model
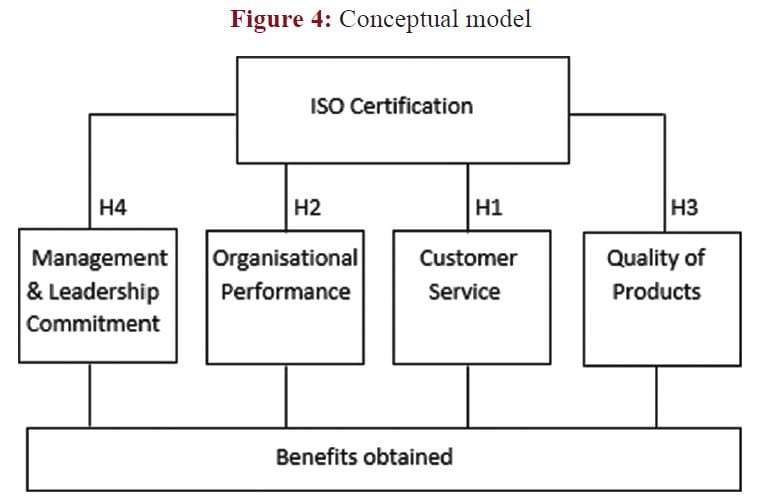
Mosadeghrad (2015) further state that leaders and managers must personally be involved in developing and implementing the quality management change and support continuous improvement.
With regards to ISO 13485:2016, top management must be committed towards the formation and implementation of the quality management system (Reid, 2020). The continual improvement and effectiveness of the quality management system is the responsibility of top management. The ISO 13485 standard stipulates that top management shall communicate the importance of customer and regulatory requirements to the entire company, establish a quality policy, make sure the quality objectives are established and conduct management reviews. Top management must provide the necessary resources required. The focus on customer requirements and applicable regulatory requirements must be determined and met by top management. Furthermore, the depth of knowledge and involvement of managers at different levels must be assessed to determine the effect of management commitment with regards to ISO certification.
Given the above theoretical arguments, to may be postulated that there is a positive relationship between the effectiveness of ISO certification and management commitment, organizational performance, customer service and product quality.
The above-mentioned postulated relationships could be depicted in the conceptual model reflected in Figure 4.
DISCUSSION AND CONCLUSION
On 9 December 2016, The Department of Health (DOH) published an update on the regulations related to in vitro diagnostics (IVD) medical devices in the Government Gazette number 40480. It stated that registration of IVD medical devices will be implemented. The DOH further stated that as part of the application process, companies must provide the Council certification to a Quality Management System for medical devices and IVDs. This means that all IVD companies must have ISO certification. It is therefore important for the IVD companies in SA to understand if the update in regulations with regards to ISO certification in actual fact will make a difference in the quality of products in the market and how it will impact the IVD industry, and in particular
the IVD companies. It is also important to understand and identify the impact, if any, on small, medium and large IVD companies in South Africa for the sustainability of products and companies in South Africa. With the introduction of mandatory ISO certification within the IVD industry in South Africa, and added to this a specific Quality Management System for Medical Devices (ISO 13485:2016), the value, benefits and sustainability of the change in the quality criteria with regards to ISO certification in the IVD industry in South Africa needs to be assessed.
The research will contribute specifically to the South African IVD market and possibly other IVD markets across the world with regards to understanding the relationship between quality and certification. Furthermore, no research to date has been performed specifically on the contribution or impact of ISO certification in the IVD industry in South Africa. It is thus unclear what impact ISO certification has on the South Africa IVD medical device industry and how the forced ISO certification will affect individual companies and the industry with regards to the quality of products and service delivery to customers.
In conclusion, if the conceptual model is to stand the test of time, it would be necessary to undertake a quantitative study to test the relationship between ISO certification and management commitment, ISO certification and organizational performance, ISO certification and customer service and ISO certification and the quality of products.





