Introduction
The updated medical device Regulations introduced mandatory certification to ISO 13485:2016 – a Medical Device (MD) and In Vitro Diagnostic (IVD) industry-specific standard.
The study was undertaken to access the impact of mandatory ISO certification within the IVD industry and to develop a theoretical framework for practical relevance.
Methods
A concurrent mixed method research approach was used to combine both a quantitative survey strategy associated with a deductive research approach, qualitative data collection techniques (interviews) and analytical procedures.
A framework was developed with constructs and their relationships with one another. Constructs measured included top management commitment, adoption of ISO certification, product quality, organisational performance and customer service.
Table 1. Path analysis results
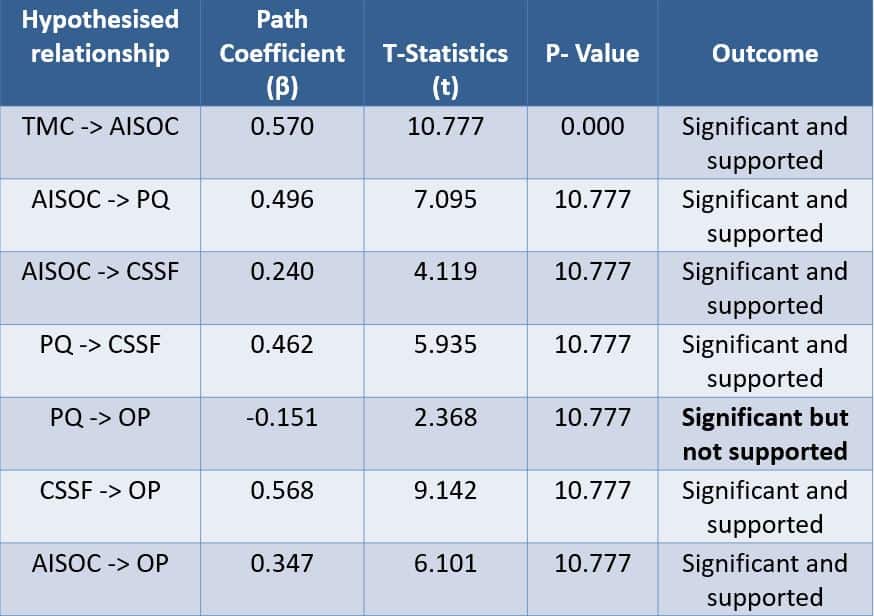
Note: AISOC= Adoption of ISO certification; CSSF= Customer service satisfaction; OP= Organisational performance; PQ= Product quality; TMC= Top management commitment
Results
As indicated in Table 1, the findings reveal that top managements’ commitment has a positive association with the adoption of ISO certification.
The adoption of ISO certification positively correlates with product quality, customer service satisfaction, as well as organisational performance. Product quality is associated with customer service satisfaction.
Contrary to what has been reported in the literature, there is a significant negative relationship between product quality and organisational performance (β= – 0.151).
It was evident that there are mixed views on whether there is a need for ISO certification in the IVD industry in South Africa.
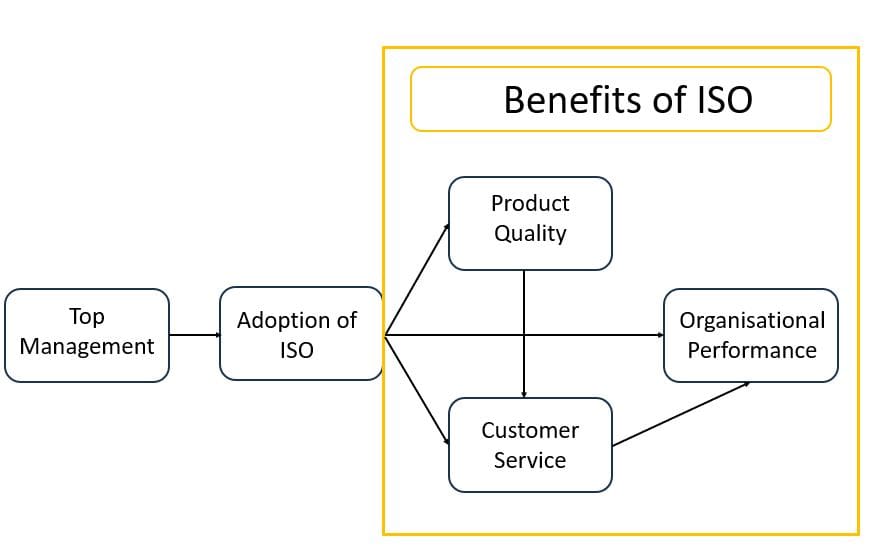
Figure 2. Theoretical Framework
Discussion
The theoretical framework indicated in Figure 2 provides managers and researchers with a better understanding of ISO certification in the IVD industry. The data provides practical relevance to the leadership, managers and policy makers in the IVD industry.
ISO certification provides many benefits (Figure 3). It assists to create a culture of continuous improvement, innovation and has enabled organisations to better manage their resources.
Additionally, it has helped to create a more transparent and accountable business environment that has helped to ensure organisations are compliant with international standards.
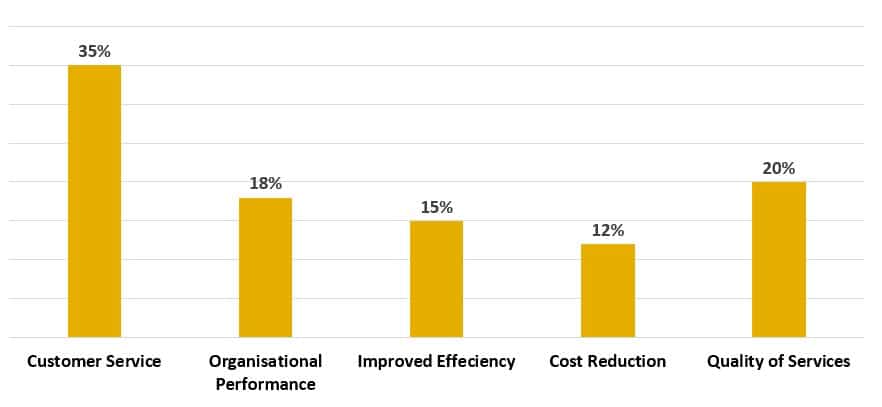
Figure 3. Benefits reported from ISO Certification
Conclusion’s
There is a definite need for ISO certification within the IVD industry in South Africa that, in turn, ensures sustainable quality and regulated products.
Top management’s involvement and commitment is essential to foster a culture of quality throughout the organisation.
It is recommended that more policy interventions should be focused on encouraging companies to adopt ISO certification as it has a strong and significant effect on product quality, customer service satisfaction and organisational performance.
Sources and reference
Dr J. C. Stoltz, PhD, K Govender, Prof Regenesys Business School, University of KwaZulu-Natal